An estriol-eluting pessary to treat pelvic organ prolapse
Preliminary optimization experiments
To guage the affect of silicone supplies on the drug launch fee and mechanical power, we ready two silicone movies, MED-4830 and MED-4870 and loaded these with totally different drug concentrations and carried out morphological evaluation and drug launch research on these samples, as detailed under.
Preparation of silicone rings
The pessary ring mannequin and the injection mould have been designed in SolidWorks computer-aided design (CAD) software program (Solidworks 2016 × 64 Version, Dassault Systemes) and exported as stereolithography (STL) information (Fig. 7). The pessary was designed as a hoop with 64 mm outer diameter, which is essentially the most generally used dimension, and a central membrane of 1 mm thickness. The injection mould was designed as half-ring form and manufactured by way of a Selective Laser Sintering (SLS) 3D printer (Formiga P100, EOS, Germany) with nylon powder (PA2200, EOS, Germany), which might tolerate the heating throughout silicone curing (160 ℃, 10 min).
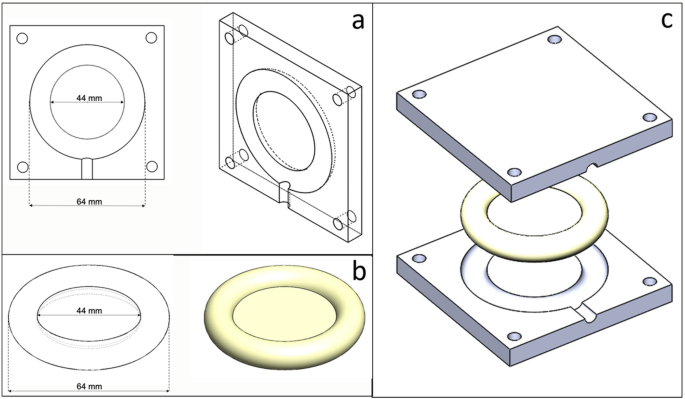
Injection mould and ring design sketch: (a) injection mould mannequin, (b) ring mannequin and (c) mould and ring stable mannequin.
Silicone supplies, MED-4830 and MED-4870, have been kindly gifted by Nusil (Carpinteria, United States). Estriol powder (Flem Pharma, Shanghai, China) at 1%,10% and 15% drug in polymer weight by weight proportion w/w (Desk 2) was weighed and step by step added and combined into the silicon. The silicon within the drug-free pessaries was ready the identical method besides estriol powder was not added. The rings have been made by filling estriol-mixed or estriol-free silicone paste into two mould halves and mounted with screws adopted by heat-introduced curing at 160 °C for 10 min after which saved at room temperature in a single day to permit correct curing of the silicone matrix. Afterwards, the samples have been faraway from the nylon molds and saved at ambient temperature for additional characterisation.
Morphology evaluation
The outer diameter of the silicone rings have been measured by an electrical Vernier caliper (TD2082, Jaycar Electronics, New Zealand). All parameters have been measured at three totally different positions of every ring and repeated in triplicate samples. The weights of the pessaries have been measured utilizing an digital steadiness (AUW220D, SHIMADZU, Japan) (n = 3). Cross-section and floor photographs have been obtained using a Schottky area emission Scanning Electron Microscopy (SEM) (SU-70, Hitachi, United Kingdom) below a working voltage of 5 kilowatt.
Estriol launch from silicone rings
In vitro estriol launch from silicone rings (MED-4830 and MED-4870) have been studied in simulated vaginal fluids in pH 4.2 (regular vaginal pH) and pH 4.5 (publish menopause vaginal pH). Samples have been precisely weighed after which positioned in a screw prime container with 200 mL simulated vaginal fluid. All samples have been shaken at 60 rpm at 35 ± 2 °C. At predetermined time intervals, 100 mL of the incubation media from every pattern was collected and an equal quantity of contemporary media was added into every launch system to keep up the full quantity and sink situations. The focus of estriol within the launch media was decided by HPLC (see description under). The cumulative quantity of estriol launched was plotted in opposition to time for every pattern (n = 3). Estriol-free rings have been used as controls. To know the mechanism of estriol launch from silicone rings, kinetics fashions have been used to analyse the discharge efficiency (as described within the following part: kinetic fashions).
The simulated vaginal fluid was ready with two pH values; pH 4.2 simulating a pre-menopausal vaginal fluid and pH 4.5 to simulate a post-menopausal vaginal fluid, in response to a technique revealed beforehand25. Potassium hydroxide (ACS reagent, ≥ 85%), calcium hydroxide (ACS reagent, ≥ 95.0%), urea (ACS reagent, 99.0–100.5%), D-( +)-Glucose (ACS reagent), glycerol (ACS reagent, ≥ 99.5%), lactic acid (meets USP testing specs), acetic acid (ACS reagent, ≥ 99.7%), and hydrochloric acid (ACS reagent, 37%) have been obtained from Sigma-Aldrich (Auckland, New Zealand). Bovine serum albumin (BSA, fatty acid free) was bought from MP Biomedicals (Auckland, New Zealand). All different used chemical substances have been analytical grade.
Estriol was measured by excessive efficiency liquid chromatography (HPLC) utilizing LC-20AT liquid chromatography LC-20AT HT auto sampler, DGU-20A5 degasser, RF-10A XL UV/VIS detector (Shimadzu USA manufacturing Inc, USA) geared up with a Phenomenex Synergi polar-RP 80A, 4.6 × 250 mm, 4 µm column. A mix of acetonitrile: 0.1% formic acid in water (50:50) was used as cellular part at a move fee of 1 mL/min and injection quantity of 10 µL with UV detection at 225 nm.
Kinetic fashions
To check the discharge kinetics and mechanism of estriol launch from the silicone movie and ring programs, knowledge was fitted to the fashions proven under:
Zero-order mannequin26:
$${Q}_{t}={Q}_{0}+{Ok}_{0}t,$$
(1)
the place ({Q}_{t}) is the quantity of drug launched at time (t), ({Q}_{0}) is the preliminary quantity of drug within the answer, and ({Ok}_{0}) is the zero-order launch fixed expressed within the models of focus/time. Zero-order mannequin describes a system the place the discharge fee of integrated drug is unbiased of its focus27.
Higuchi mannequin26:
$${mathrm{Q}}_{t}={Ok}_{H}instances {t}^{1/2},$$
(2)
the place ({mathrm{Q}}_{t}) is quantity of drug launched in time (t), ({Ok}_{H}) is the Higuchi dissolution fixed expressed within the models of focus/time. Higuchi mannequin is proposed to explain the drug launch from a matrix system with totally different geometrics and porous construction28.
Mechanical check
Compression check of the silicone rings have been carried out utilizing a texture analyser (TA.XT2, Secure Micro System, Haslemere, Surrey, UK) by compressing the ring for a distance of 10 mm (n ≥ 3 compressions at totally different websites per ring) and the utmost compression power in every check was recorded20. Drug-free and drug-loaded rings have been in comparison with decide whether or not drug incorporation (and the relative focus loaded) impacts the mechanical property of the rings. Contemplating the rings are designed for long-term software, the rings handled with SVF for a interval of 4-months at 35 ± 2 °C have been assessed to guage the change of mechanical power throughout remedy. A business ring (Milex/Cooper Surgical) was used as a reference. Every ring was positioned vertically on a hoop holder mounted to the platform of the feel analyser. A probe hooked up to the movable arm was used to compress the ring at a distance of 10 mm at a pace of two.0 mm/s and the utmost compression forces have been recorded.
Aspect presence and chemical interactions
In an effort to establish the drug presence within the silicone matrix and to grasp if there may be any interactions between estriol and silicone or chemical construction adjustments within the preparation remedy, spectra obtained from Fourier-transform infrared (FTIR) spectroscopy have been used to characterise the presence of particular chemical teams of estriol and silicones and their doable interactions. This was completed utilizing a Nicolet iS10 FTIR spectrophotometer (Thermo Scientific, USA). FTIR is used to establish totally different compounds and their interactions.
Statistical evaluation
A minimal of three technical triplicates have been carried out for every in vitro research. Information have been subjected to one-way evaluation of variant (ANOVA) with the extent of significance set at p < 0.05.